Glycated hemoglobin
| Glycated hemoglobin | |
|---|---|
| MedlinePlus | 003640 |
| eMedicine | 2049478 |
| LOINC | 41995-2 |
Glycated hemoglobin (also called glycohemoglobin or glycosylated hemoglobin) is a form of hemoglobin (Hb) that is chemically linked to a sugar.
Most monosaccharides, including glucose, galactose, and fructose, spontaneously (that is, non-enzymatically) bond with hemoglobin when they are present in the bloodstream. However, glucose is only 21% as likely to do so as galactose and 13% as likely to do so as fructose, which may explain why glucose is used as the primary metabolic fuel in humans.[1][2]
The formation of excess sugar-hemoglobin linkages indicates the presence of excessive sugar in the bloodstream and is an indicator of diabetes or other hormone diseases in high concentration (HbA1c >6.4%).[3] A1c is of particular interest because it is easy to detect. The process by which sugars attach to hemoglobin is called glycation and the reference system is based on HbA1c, defined as beta-N-1-deoxy fructosyl hemoglobin as component.[4]
There are several ways to measure glycated hemoglobin, of which HbA1c (or simply A1c) is a standard single test.[5] HbA1c is measured primarily to determine the three-month average blood sugar level and is used as a standard diagnostic test for evaluating the risk of complications of diabetes and as an assessment of glycemic control.[5][6] The test is considered a three-month average because the average lifespan of a red blood cell is three to four months. Normal levels of glucose produce a normal amount of glycated hemoglobin. As the average amount of plasma glucose increases, the fraction of glycated hemoglobin increases in a predictable way. In diabetes, higher amounts of glycated hemoglobin, indicating higher of blood glucose levels, have been associated with cardiovascular disease, nephropathy, neuropathy, and retinopathy.[7]
Terminology
[edit]Glycated hemoglobin is preferred over glycosylated hemoglobin to reflect the correct (non-enzymatic) process. Early literature often used glycosylated as it was unclear which process was involved until further research was performed. The terms are still sometimes used interchangeably in English-language literature.[8]
The naming of HbA1c derives from hemoglobin type A being separated on cation exchange chromatography. The first fraction to separate, probably considered to be pure hemoglobin A, was designated HbA0, and the following fractions were designated HbA1a, HbA1b, and HbA1c, in their order of elution. Improved separation techniques have subsequently led to the isolation of more subfractions.[9]
History
[edit]Hemoglobin A1c was first separated from other forms of hemoglobin by Huisman and Meyering in 1958 using a chromatographic column.[10] It was first characterized as a glycoprotein by Bookchin and Gallop in 1968.[11] Its increase in diabetes was first described in 1969 by Samuel Rahbar et al.[12] The reactions leading to its formation were characterized by Bunn and his coworkers in 1975.[13]
The use of hemoglobin A1c for monitoring the degree of control of glucose metabolism in diabetic patients was proposed in 1976 by Anthony Cerami, Ronald Koenig, and coworkers.[14]
Damage mechanisms
[edit]Glycated hemoglobin causes an increase of highly reactive free radicals inside blood cells, altering the properties of their cell membranes. This leads to blood cell aggregation and increased blood viscosity, which results in impaired blood flow.[15]
Another way glycated hemoglobin causes damage is via inflammation, which results in atherosclerotic plaque (atheroma) formation. Free-radical build-up promotes the excitation of Fe2+-hemoglobin through Fe3+-Hb into abnormal ferryl hemoglobin (Fe4+-Hb). Fe4+ is unstable and reacts with specific amino acids in hemoglobin to regain its Fe3+ oxidation state. Hemoglobin molecules clump together via cross-linking reactions, and these hemoglobin clumps (multimers) promote cell damage and the release of Fe4+-hemoglobin into the matrix of innermost layers (subendothelium) of arteries and veins. This results in increased permeability of interior surface (endothelium) of blood vessels and production of pro-inflammatory monocyte adhesion proteins, which promote macrophage accumulation in blood vessel surfaces, ultimately leading to harmful plaques in these vessels.[15]
Highly glycated Hb-AGEs go through vascular smooth muscle layer and inactivate acetylcholine-induced endothelium-dependent relaxation, possibly through binding to nitric oxide (NO), preventing its normal function. NO is a potent vasodilator and also inhibits formation of plaque-promoting LDLs (sometimes called "bad cholesterol") oxidized form.[15]
This overall degradation of blood cells also releases heme from them. Loose heme can cause oxidation of endothelial and LDL proteins, which results in plaques.[15]
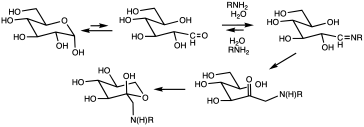
Principle in medical diagnostics
[edit]Glycation of proteins is a frequent occurrence, but in the case of hemoglobin, a nonenzymatic condensation reaction occurs between glucose and the N-end of the beta chain. This reaction produces a Schiff base (R−N=CHR', R=beta chain, CHR'=glucose-derived), which is itself converted to 1-deoxyfructose. This second conversion is an example of an Amadori rearrangement.[citation needed]
When blood glucose levels are high, glucose molecules attach to the hemoglobin in red blood cells. The longer hyperglycemia occurs in blood, the more glucose binds to hemoglobin in the red blood cells and the higher the glycated hemoglobin.[17]
Once a hemoglobin molecule is glycated, it remains that way. A buildup of glycated hemoglobin within the red cell, therefore, reflects the average level of glucose to which the cell has been exposed during its life-cycle. Measuring glycated hemoglobin assesses the effectiveness of therapy by monitoring long-term serum glucose regulation.
A1c is a weighted average of blood glucose levels during the life of the red blood cells (117 days for men and 106 days in women[18]). Therefore, glucose levels on days nearer to the test contribute substantially more to the level of A1c than the levels in days further from the test.[19]
This is also supported by data from clinical practice showing that HbA1c levels improved significantly after 20 days from start or intensification of glucose-lowering treatment.[20]
Measurement
[edit]Several techniques are used to measure hemoglobin A1c. Laboratories may use high-performance liquid chromatography, immunoassay, enzymatic assay, capillary electrophoresis, or boronate affinity chromatography. Point of care (e.g., doctor's office) devices use immunoassay boronate affinity chromatography.[17]
In the United States, HbA1c testing laboratories are certified by the National Glycohemoglobin Standardization Program to standardize them against the results of the 1993 Diabetes Control and Complications Trial (DCCT).[21] An additional percentage scale, Mono S has previously been in use by Sweden and KO500 is in use in Japan.[22][23]
Switch to IFCC units
[edit]The American Diabetes Association, European Association for the Study of Diabetes, and International Diabetes Federation have agreed that, in the future, HbA1c is to be reported in the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) units.[24] IFCC reporting was introduced in Europe except for the UK in 2003;[25] the UK carried out dual reporting from 1 June 2009 [26] until 1 October 2011.
Conversion between DCCT and IFCC is by the following equation:[27]
| "IFCC" HbA1c | "DCCT" HbA1c | "Mono S" HbA1c[23] |
|---|---|---|
| (mmol/mol) | (%) | (%) |
| 10 | 3.1 | 2.0 |
| 20 | 4.0 | 2.9 |
| 30 | 4.9 | 3.9 |
| 40 | 5.8 | 4.8 |
| 45 | 6.3 | 5.3 |
| 50 | 6.7 | 5.8 |
| 55 | 7.2 | 6.3 |
| 60 | 7.6 | 6.8 |
| 65 | 8.1 | 7.2 |
| 70 | 8.6 | 7.7 |
| 80 | 9.5 | 8.7 |
| 90 | 10.4 | 9.6 |
| 100 | 11.3 | 10.6 |
Interpretation of results
[edit]Laboratory results may differ depending on the analytical technique, the age of the subject, and biological variation among individuals. Higher levels of HbA1c are found in people with persistently elevated blood sugar, as in diabetes mellitus. While diabetic patient treatment goals vary, many include a target range of HbA1c values. A diabetic person with good glucose control has an HbA1c level that is close to or within the reference range.[citation needed]
The International Diabetes Federation and the American College of Endocrinology recommend HbA1c values below 48 mmol/mol (6.5 DCCT %), while the American Diabetes Association recommends HbA1c be below 53 mmol/mol (7.0 DCCT %) for most patients.[28] Results from large trials in 2008–09 suggested that a target below 53 mmol/mol (7.0 DCCT %) for older adults with type 2 diabetes may be excessive: Below 53 mmol/mol, the health benefits of reduced A1c become smaller, and the intensive glycemic control required to reach this level leads to an increased rate of dangerous hypoglycemic episodes.[29]
A retrospective study of 47,970 type 2 diabetes patients, aged 50 years and older, found that patients with an HbA1c more than 48 mmol/mol (6.5 DCCT %) had an increased mortality rate,[30] but a later international study contradicted these findings.[31][32][33]
A review of the UKPDS, Action to Control Cardiovascular Risk in Diabetes (ACCORD), Advance and Veterans Affairs Diabetes Trials (VADT) estimated that the risks of the main complications of diabetes (diabetic retinopathy, diabetic nephropathy, diabetic neuropathy, and macrovascular disease) decreased by about 3% for every 1 mmol/mol decrease in HbA1c.[34]
However, a trial by ACCORD designed specifically to determine whether reducing HbA1c below 42 mmol/mol (6.0 DCCT %) using increased amounts of medication would reduce the rate of cardiovascular events found higher mortality with this intensive therapy, so much so that the trial was terminated 17 months early.[35]
Practitioners must consider patients' health, their risk of hypoglycemia, and their specific health risks when setting a target HbA1c level. Because patients are responsible for averting or responding to their own hypoglycemic episodes, their input and the doctors' assessments of the patients' self-care skills are also important.[citation needed]
Persistent elevations in blood sugar (and, therefore, HbA1c) increase the risk of long-term vascular complications of diabetes, such as coronary disease, heart attack, stroke, heart failure, kidney failure, blindness, erectile dysfunction, neuropathy (loss of sensation, especially in the feet), gangrene, and gastroparesis (slowed emptying of the stomach). Poor blood glucose control also increases the risk of short-term complications of surgery such as poor wound healing.[citation needed]
All-cause mortality is higher above 64 mmol/mol (8.0 DCCT%) HbA1c as well as below 42 mmol/mol (6.0 DCCT %) in diabetic patients, and above 42 mmol/mol (6.0 DCCT %) as well as below 31 mmol/mol (5.0 DCCT %) in non-diabetic persons, indicating the risks of hyperglycemia and hypoglycemia, respectively.[7] Similar risk results are seen for cardiovascular disease.[7]
The 2022 ADA guidelines reaffirmed the recommendation that HbA1c should be maintained below 7.0% for most patients. Higher target values are appropriate for children and adolescents, patients with extensive co-morbid illness and those with a history of severe hypoglycemia. More stringent targets (<6.0%) are preferred for pregnant patients if this can be achieved without significant hypoglycemia.[36]
Factors other than glucose that affect A1c
[edit]Lower-than-expected levels of HbA1c can be seen in people with shortened red blood cell lifespans, such as with glucose-6-phosphate dehydrogenase deficiency, sickle-cell disease, or any other condition causing premature red blood cell death. For these patients, alternate assessment with fructosamine or glycated albumin is recommended; these methods reflect glycemic control over the preceding 2-3 weeks.[37] Blood donation will result in rapid replacement of lost RBCs with newly formed red blood cells. Since these new RBCs will have only existed for a short period of time, their presence will lead HbA1c to underestimate the actual average levels. There may also be distortions resulting from blood donation during the preceding two months, due to an abnormal synchronization of the age of the RBCs, resulting in an older than normal average blood cell life (resulting in an overestimate of actual average blood glucose levels). Conversely, higher-than-expected levels can be seen in people with a longer red blood cell lifespan, such as with iron deficiency.[38]
Results can be unreliable in many circumstances, for example after blood loss, after surgery, blood transfusions, anemia, or high erythrocyte turnover; in the presence of chronic renal or liver disease; after administration of high-dose vitamin C; or erythropoetin treatment.[39] Hypothyroidism can artificially raise the A1c.[40][41][42] In general, the reference range (that found in healthy young persons), is about 30–33 mmol/mol (4.9–5.2 DCCT %).[43] The mean HbA1c for diabetics type 1 in Sweden in 2014 was 63 mmol/mol (7.9 DCCT%) and for type 2, 61 mmol/mol (7.7 DCCT%).[44] HbA1c levels show a small, but statistically significant, progressive uptick with age; the clinical importance of this increase is unclear.[37]
Mapping from A1c to estimated average glucose
[edit]The approximate mapping between HbA1c values given in DCCT percentage (%) and eAG (estimated average glucose) measurements is given by the following equation:[39]
- eAG(mg/dL) = 28.7 × A1C − 46.7
eAG(mmol/L) = 1.59 × A1C − 2.59
(Data in parentheses are 95% confidence intervals>)
| HbA1c | eAG | ||
|---|---|---|---|
| % | mmol/mol[45] | mmol/L | mg/dL |
| 5 | 31 | 5.4 (4.2–6.7) | 97 (76–120) |
| 6 | 42 | 7.0 (5.5–8.5) | 126 (100–152) |
| 7 | 53 | 8.6 (6.8–10.3) | 154 (123–185) |
| 8 | 64 | 10.2 (8.1–12.1) | 183 (147–217) |
| 9 | 75 | 11.8 (9.4–13.9) | 212 (170–249) |
| 10 | 86 | 13.4 (10.7–15.7) | 240 (193–282) |
| 11 | 97 | 14.9 (12.0–17.5) | 269 (217–314) |
| 12 | 108 | 16.5 (13.3–19.3) | 298 (240–347) |
| 13 | 119 | 18.1 (15–21) | 326 (260–380) |
| 14 | 130 | 19.7 (16–23) | 355 (290–410) |
| 15 | 140 | 21.3 (17–25) | 384 (310–440) |
| 16 | 151 | 22.9 (19–26) | 413 (330–480) |
| 17 | 162 | 24.5 (20–28) | 441 (460–510) |
| 18 | 173 | 26.1 (21–30) | 470 (380–540) |
| 19 | 184 | 27.7 (23–32) | 499 (410–570) |
Normal, prediabetic, and diabetic ranges
[edit]The 2010 American Diabetes Association Standards of Medical Care in Diabetes added the HbA1c ≥ 48 mmol/mol (≥6.5 DCCT %) as another criterion for the diagnosis of diabetes.[46]
| Diagnosis | "IFCC" HbA1c | "DCCT" HbA1c | "Mono S" HbA1c |
|---|---|---|---|
| Normal | <40 mmol/mol | <5.7% | <4.7% |
| Prediabetes | 40–47 mmol/mol | 5.7–6.4% | 4.7–5.4% |
| Diabetes | ≥48 mmol/mol | ≥6.5% | >5.5% |
Indications and uses
[edit]Glycated hemoglobin testing is recommended for both checking the blood sugar control in people who might be prediabetic and monitoring blood sugar control in patients with more elevated levels, termed diabetes mellitus. For a single blood sample, it provides far more revealing information on glycemic behavior than a fasting blood sugar value. However, fasting blood sugar tests are crucial in making treatment decisions. The American Diabetes Association guidelines are similar to others in advising that the glycated hemoglobin test be performed at least twice a year in patients with diabetes who are meeting treatment goals (and who have stable glycemic control) and quarterly in patients with diabetes whose therapy has changed or who are not meeting glycemic goals.[48][36]
Glycated hemoglobin measurement is not appropriate where a change in diet or treatment has been made within six weeks. Likewise, the test assumes a normal red blood cell aging process and mix of hemoglobin subtypes (predominantly HbA in normal adults). Hence, people with recent blood loss, hemolytic anemia, or genetic differences in the hemoglobin molecule (hemoglobinopathy) such as sickle-cell disease and other conditions, as well as those who have donated blood recently, are not suitable for this test.[49]
Due to glycated hemoglobin's variability (as shown in the table above), additional measures should be checked in patients at or near recommended goals. People with HbA1c values at 64 mmol/mol or less should be provided additional testing to determine whether the HbA1c values are due to averaging out high blood glucose (hyperglycemia) with low blood glucose (hypoglycemia) or the HbA1c is more reflective of an elevated blood glucose that does not vary much throughout the day. Devices such as continuous blood glucose monitoring allow people with diabetes to determine their blood glucose levels on a continuous basis, testing every few minutes. Continuous use of blood glucose monitors is becoming more common, and the devices are covered by many health insurance plans, including Medicare in the United States. The supplies tend to be expensive, since the sensors must be changed at least every 2 weeks. Another useful test in determining if HbA1c values are due to wide variations of blood glucose throughout the day is 1,5-anhydroglucitol, also known as GlycoMark. GlycoMark reflects only the times that the person experiences hyperglycemia above 180 mg/dL over a two-week period.[citation needed]
Concentrations of hemoglobin A1 (HbA1) are increased, both in diabetic patients and in patients with kidney failure, when measured by ion-exchange chromatography. The thiobarbituric acid method (a chemical method specific for the detection of glycation) shows that patients with kidney failure have values for glycated hemoglobin similar to those observed in normal subjects, suggesting that the high values in these patients are a result of binding of something other than glucose to hemoglobin.[50]
In autoimmune hemolytic anemia, concentrations of HbA1 is undetectable. Administration of prednisolone will allow the HbA1 to be detected.[51] The alternative fructosamine test may be used in these circumstances and it also reflects an average of blood glucose levels over the preceding 2 to 3 weeks.[52]
All the major institutions such as the International Expert Committee Report, drawn from the International Diabetes Federation, the European Association for the Study of Diabetes, and the American Diabetes Association, suggest the HbA1c level of 48 mmol/mol (6.5 DCCT %) as a diagnostic level.[53] The Committee Report further states that, when HbA1c testing cannot be done, the fasting and glucose-tolerance tests be done. Screening for diabetes during pregnancy continues to require fasting and glucose-tolerance measurements for gestational diabetes at 24 to 28 weeks gestation, although glycated hemoglobin may be used for screening at the first prenatal visit.[37]
Modification by diet
[edit]Meta-analysis has shown probiotics to cause a statistically significant reduction in glycated hemoglobin in type-2 diabetics.[54] Trials with multiple strains of probiotics had statistically significant reductions in glycated hemoglobin, whereas trials with single strains did not.[54]
Standardization and traceability
[edit]Most clinical studies recommend the use of HbA1c assays that are traceable to the DCCT assay.[55] The National Glycohemoglobin Standardization Program (NGSP) and IFCC have improved assay standardization.[37] For initial diagnosis of diabetes, only HbA1c methods that are NGSP-certified should be used, not point-of-care testing devices.[36] Analytical performance has been a problem with earlier point-of-care devices for HbA1c testing, specifically large standard deviations and negative bias.[37]
Veterinary medicine
[edit]HbA1c testing has not been found useful in the monitoring during the treatment of cats and dogs with diabetes, and is not generally used; monitoring of fructosamine levels is favoured instead.[56]
See also
[edit]References
[edit]- ^ Bunn HF, Higgins PJ (July 1981). "Reaction of monosaccharides with proteins: possible evolutionary significance". Science. 213 (4504): 222–4. Bibcode:1981Sci...213..222B. doi:10.1126/science.12192669. PMID 12192669.
- ^ McPherson JD, Shilton BH, Walton DJ (March 1988). "Role of fructose in glycation and cross-linking of proteins". Biochemistry. 27 (6): 1901–7. doi:10.1021/bi00406a016. PMID 3132203.
- ^ Pongudom, Saranya (1 November 2019). "Determination of Normal HbA1C Levels in Non-Diabetic Patients with Hemoglobin E". Annals of Clinical & Laboratory Science. 49 (6): 804–9. PMID 31882432.
- ^ Miedema K (2005). "Standardization of HbA1c and Optimal Range of Monitoring". Scandinavian Journal of Clinical and Laboratory Investigation. 240: 61–72. doi:10.1080/00365510500236143. PMID 16112961. S2CID 30162967.
- ^ a b Elizabeth Weiser Caswell Diabetes Institute. Hemoglobin A1c Fact Sheet. Accessed 2024-07-02.
- ^ "2. Glycated haemoglobin (HbA1c) for the diagnosis of diabetes". Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. WHO Guidelines Approved by the Guidelines Review Committee. World Health Organization. 2011. PMID 26158184. NBK304271.
- ^ a b c Cavero-Redondo I, Peleteiro B, Martínez-Vizcaíno V (2017). "Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis". BMJ Open. 7 (7): e015949. doi:10.1136/bmjopen-2017-015949. PMC 5642750. PMID 28760792.
- ^ Oliwia Witczak, Trine B. Haugen (25 November 2014). "Glycated or glycosylated?". Journal of the Norwegian Medical Association. 134 (22): 2179. doi:10.4045/tidsskr.14.0172. PMID 25423986. Archived from the original on 5 December 2018. Retrieved 5 December 2018.
Hospitals should ensure that the correct term for HbA1c — glycated haemoglobin — is now to be found in laboratory manuals.
- ^ Peterson KP, Pavlovich JG, Goldstein D, Little R, England J, Peterson CM (1998). "What is hemoglobin A1c? An analysis of glycated hemoglobins by electrospray ionization mass spectrometry". Clinical Chemistry. 44 (9): 1951–8. doi:10.1093/clinchem/44.9.1951. PMID 9732983. Archived from the original on 2015-09-23. Retrieved 2024-06-21.
- ^ Huisman TH, Martis EA, Dozy A (1958). "Chromatography of hemoglobin types on carboxymethylcellulose". J. Lab. Clin. Med. 52 (2): 312–327. PMID 13564011.
- ^ Bookchin RM, Gallop PM (1968). "Structure of haemoglobin A1c: nature of the N-terminal beta chain blocking group". Biochem. Biophys. Res. Commun. 32 (1): 86–93. doi:10.1016/0006-291X(68)90430-0. PMID 4874776.
- ^ Rahbar S, Blumenfeld O, Ranney HM (1969). "Studies of an unusual hemoglobin in patients with diabetes mellitus". Biochem. Biophys. Res. Commun. 36 (5): 838–843. doi:10.1016/0006-291X(69)90685-8. PMID 5808299.
- ^ Bunn HF, Haney DN, Gabbay KH, Gallop PM (1975). "Further identification of the nature and linkage of the carbohydrate in haemoglobin A1c". Biochem. Biophys. Res. Commun. 67 (1): 103–9. doi:10.1016/0006-291X(75)90289-2. PMID 1201013.
- ^ Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A (1976). "Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus". N. Engl. J. Med. 295 (8): 417–420. doi:10.1056/NEJM197608192950804. PMID 934240.
- ^ a b c d Saleh, Jumana (2015-08-26). "Glycated hemoglobin and its spinoffs: Cardiovascular disease markers or risk factors?". World Journal of Cardiology. 7 (8): 449–453. doi:10.4330/wjc.v7.i8.449. PMC 4549778. PMID 26322184.
- ^ Yaylayan, Varoujan A.; Huyghues-Despointes, Alexis (1994). "Chemistry of Amadori Rearrangement Products: Analysis, Synthesis, Kinetics, Reactions, and Spectroscopic Properties". Critical Reviews in Food Science and Nutrition. 34 (4): 321–369. doi:10.1080/10408399409527667. PMID 7945894.
- ^ a b Pohanka M (March 2021). "Glycated Hemoglobin and Methods for Its Point of Care Testing". Biosensors. 11 (3): 70. doi:10.3390/bios11030070. PMC 8000313. PMID 33806493.
- ^ Unnikrishnan, Ranjit (Jul–Aug 2012). "Drugs affecting HbA1c levels". Indian Journal of Endocrinology and Metabolism. 16 (4): 528–531. doi:10.4103/2230-8210.98004. PMC 3401751. PMID 22837911.
- ^ "NGSP: HbA1c and eAG". ngsp.org. Archived from the original on 2015-10-15. Retrieved 2024-06-21.
- ^ Sidorenkov G, Haaijer-Ruskamp FM, de Zeeuw D, Denig P (2011). "A longitudinal study examining adherence to guidelines in diabetes care according to different definitions of adequacy and timeliness". PLOS ONE. 6 (9): e24278. Bibcode:2011PLoSO...624278S. doi:10.1371/journal.pone.0024278. PMC 3169586. PMID 21931669.
- ^ Developing Point of care HbA1c tests for Diabetes monitoring Archived 2008-08-29 at the Wayback Machine, Barry Plant, Originally Published IVDT July/August 2008
- ^ [Clinical Chemistry 50:1 166–174 (2004)]
- ^ a b HbA1c in a new way Archived 2013-09-09 at the Wayback Machine By the Swedish Diabetes Association. Retrieved 2023-02-01.
- ^ Geistanger A, Arends S, Berding C, Hoshino T, Jeppsson JO, Little R, Siebelder C, Weykamp C (August 2008). "Statistical methods for monitoring the relationship between the IFCC reference measurement procedure for hemoglobin A1c and the designated comparison methods in the United States, Japan, and Sweden". Clin. Chem. 54 (8): 1379–85. doi:10.1373/clinchem.2008.103556. PMID 18539643.
- ^ Manley S, John WG, Marshall S (July 2004). "Introduction of IFCC reference method for calibration of HbA: implications for clinical care". Diabet. Med. 21 (7): 673–6. doi:10.1111/j.1464-5491.2004.01311.x. PMID 15209757. S2CID 30468208.
- ^ "Standardisation of the reference method for the measurement of HbA1c to improve diabetes care" (PDF) (Press release). Association for Clinical Biochemistry and Laboratory Medicine (with Diabetes UK). April 2008. Archived from the original (PDF) on 2011-07-22. Retrieved 2009-07-02.
- ^ "HbA1c Standardisation For Laboratory Professionals" (PDF). Diabetes UK (with Association for Clinical Biochemistry and Laboratory Medicine). Archived (PDF) from the original on 2011-07-20. Retrieved 2009-07-02.
- ^ "Executive Summary: Standards of medical care in diabetes — 2009". Diabetes Care. 32 (Suppl 1): S6–S12. 2009. doi:10.2337/dc09-S006. PMC 2613586. PMID 19118288.
- ^ Lehman R, Krumholz HM (2009). "Tight control of blood glucose in long standing type 2 diabetes". Br Med J. 338: b800. doi:10.1136/bmj.b800. PMID 19264821. S2CID 45188963.
- ^ Currie, Craig J; Peters, John R; Tynan, Aodán; Evans, Marc; Heine, Robert J; Bracco, Oswaldo L; Zagar, Tony; Poole, Chris D (2010). "Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study". The Lancet. 375 (9713): 481–9. doi:10.1016/S0140-6736(09)61969-3. PMID 20110121. S2CID 21223855.
- ^ "Advance Study Contradicts ACCORD Findings". Diabetes Self-Management. 2008-03-07. Archived from the original on 2012-07-17. Retrieved 2013-06-10.
- ^ ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F (June 2008). "Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes". N Engl J Med. 358 (24): 2560–72. doi:10.1056/NEJMoa0802987. PMID 18539916.
Conclusions: A strategy of intensive glucose control, involving gliclazide (modified release) and other drugs as required, that lowered the glycated hemoglobin value to 6.5% yielded a 10% relative reduction in the combined outcome of major macrovascular and microvascular events, primarily as a consequence of a 21% relative reduction in nephropathy
(Clinical trial number NCT00145925 for "Blood Pressure and Glucose Lowering for the Prevention of Vascular Disease in High Risk Patients With Type 2 Diabetes" at ClinicalTrials.gov) - ^ Heller, Simon R. (2009-11-01). "A Summary of the Advance Trial". Diabetes Care. 32 (Suppl 2): S357–61. doi:10.2337/dc09-S339. PMC 2811451. PMID 19875581.
- ^ Shubrook JH, Shubrook J (2010). "Risks and benefits of attaining HbA(1c) goals: Examining the evidence". The Journal of the American Osteopathic Association. 110 (7 Suppl 7): e7–e12. PMID 20644204.
- ^ Gerstein HC, Miller ME, Byington RP, et al. (2008). "Effects of Intensive Glucose Lowering in Type 2 Diabetes". New England Journal of Medicine. 358 (24): 2545–59. doi:10.1056/NEJMoa0802743. PMC 4551392. PMID 18539917.
- ^ a b c American Diabetes Association Professional Practice Committee (January 2022). "6. Glycemic Targets: Standards of Medical Care in Diabetes-2022". Diabetes Care. 45 (Suppl 1): S83–S96. doi:10.2337/dc22-S006. PMID 34964868.
- ^ a b c d e Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Lernmark Å, Metzger BE, Nathan DM, Sue Kirkman M (August 2023). "Executive Summary: Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus". Clin Chem. 69 (8): 777–784. doi:10.1093/clinchem/hvad079. PMID 37562009.
- ^ Kilpatrick ES, Bloomgarden ZT, Zimmet PZ (2009). "Is haemoglobin A1c a step forward for diagnosing diabetes?". BMJ. 339: b4432. doi:10.1136/bmj.b4432. PMID 19903702. S2CID 36941786.
- ^ a b Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ (2008). "Translating the A1C assay into estimated average glucose values". Diabetes Care. 31 (8): 1473–8. doi:10.2337/dc08-0545. PMC 2742903. PMID 18540046. Archived from the original on 2012-02-19. Retrieved 2009-09-24.
- ^ "Hypothyroidism Falsely Raises HbA1c and Glycated Albumin Levels". Diabetes In Control. 12 November 2010. Archived from the original on 23 September 2015. Retrieved 6 August 2015.
- ^ Kim, M. K.; Kwon, H. S.; Baek, K. H.; Lee, J. H.; Park, W. C.; Sohn, H. S.; Lee, K. W.; Song, K. H. (7 September 2010). "Effects of Thyroid Hormone on A1C and Glycated Albumin Levels in Nondiabetic Subjects With Overt Hypothyroidism". Diabetes Care. 33 (12): 2546–8. doi:10.2337/dc10-0988. PMC 2992186. PMID 20823345.
- ^ Bhattacharjee R, Thukral A, Chakraborty PP, Roy A, Goswami S, Ghosh S, Mukhopadhyay P, Mukhopadhyay S, Chowdhury S (2017). "Effects of thyroid status on glycated hemoglobin". Indian J Endocrinol Metab. 21 (1): 26–30. doi:10.4103/2230-8210.196017. PMC 5240076. PMID 28217494.
- ^ Saaddine, Jinan B.; Fagot-Campagna, Anne; Rolka, Deborah; Narayan, K. M. Venkat; Geiss, Linda; Eberhardt, Mark; Flegal, Katherine M. (2002-08-01). "Distribution of HbA(1c) levels for children and young adults in the U.S.: Third National Health and Nutrition Examination Survey". Diabetes Care. 25 (8): 1326–30. doi:10.2337/diacare.25.8.1326. ISSN 0149-5992. PMID 12145229.
- ^ "Nationella Diabetesregistret Årsrapport 2014 års resultat" (PDF). Nationella Diabetesregistret Årsrapport 2014 års resultat (in Swedish). Nationella Diabetes Registret. Archived (PDF) from the original on 2017-10-02. Retrieved 2015-12-14.
- ^ "Change to HbA1c values". Diabetes UK. 2013. Archived from the original on 2013-07-26.
- ^ "Executive summary: Standards of medical care in diabetes — 2010". Diabetes Care. 33 (Suppl 1): S4–S10. January 2010. doi:10.2337/dc10-S004. PMC 2797389. PMID 20042774. Archived from the original on 2010-01-13. Retrieved 2010-01-02.
- ^ "Diagnosing Diabetes and Learning About Prediabetes". American Diabetes Association. Archived from the original on 28 July 2017. Retrieved 2 December 2018.
- ^ American Diabetes Association (2007). "Standards of medical care in diabetes". Diabetes Care. 30 (Suppl 1): S4–S41. doi:10.2337/dc07-S004. PMID 17192377.
- ^ Klonoff, David C. (2019-03-22). "Hemoglobinopathies and Hemoglobin A1c in Diabetes Mellitus". Journal of Diabetes Science and Technology. 14 (1): 3–7. doi:10.1177/1932296819841698. PMC 7189151. PMID 30897962.
- ^ Bannon P, Lessard F, Lepage R, Joly JG, Dufresne L (March 1984). "Glycated hemoglobin in uremic patients as measured by affinity and ion-exchange chromatography". Clin Chem. 30 (3): 485–6. doi:10.1093/clinchem/30.3.485. PMID 6697506.
- ^ "Undetectable Glycosylated Hemoglobin in Autoimmune Hemolytic Anemia" (PDF). repository.oai.yamaguchi-u.ac.jp. Archived (PDF) from the original on 2011-07-16. Retrieved 2009-08-31.
- ^ Freitas PA, Ehlert LR, Camargo JL (2017). "Glycated albumin: a potential biomarker in diabetes". Arch Endocrinol Metab. 61 (3): 296–304. doi:10.1590/2359-3997000000272. PMC 10118799. PMID 28699985.
- ^ The International Expert Committee (2009). "International expert committee report on the role of the A1C assay in the diagnosis of diabetes". Diabetes Care. 32 (7): 1327–34. doi:10.2337/dc09-9033. PMC 2699715. PMID 19502545.
- ^ a b Sun J, Buys NJ (2016). "Glucose- and glycaemic factor-lowering effects of probiotics on diabetes: a meta-analysis of randomised placebo-controlled trials". British Journal of Nutrition. 115 (7): 1167–77. doi:10.1017/S0007114516000076. PMID 26899960.
- ^ Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C (September 1993). "The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus". N Engl J Med. 329 (14): 977–986. doi:10.1056/NEJM199309303291401. PMID 8366922.
- ^ Delack JB, Stogdale L (October 1983). "Glycosylated hemoglobin measurement in dogs and cats: implications for its utility in diabetic monitoring". Can Vet J. 24 (10): 308–311. PMC 1790442. PMID 17422317.
External links
[edit]- Health Information: Diabetes — National Institutes of Health (NIH): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- National Diabetes Information Clearinghouse — NIDDK (old site, archived 2010-02-21)
- Standards of Care in Diabetes, American Diabetes Association Professional Practice Committee
- Standards of Care in Diabetes — 2024 (pdf), American Diabetes Association Professional Practice Committee

![{\displaystyle \mathrm {IFCC\ HBA1c} \,{\Big (}{\frac {\text{mmol}}{\text{mol}}}{\Big )}=[\mathrm {DCCT\ HBA1c} \,(\%)-2.14]\times 10.929}](https://wikimedia.org/api/rest_v1/media/math/render/svg/89c3676c7dafce04a04309dc594afa937fab12cc)